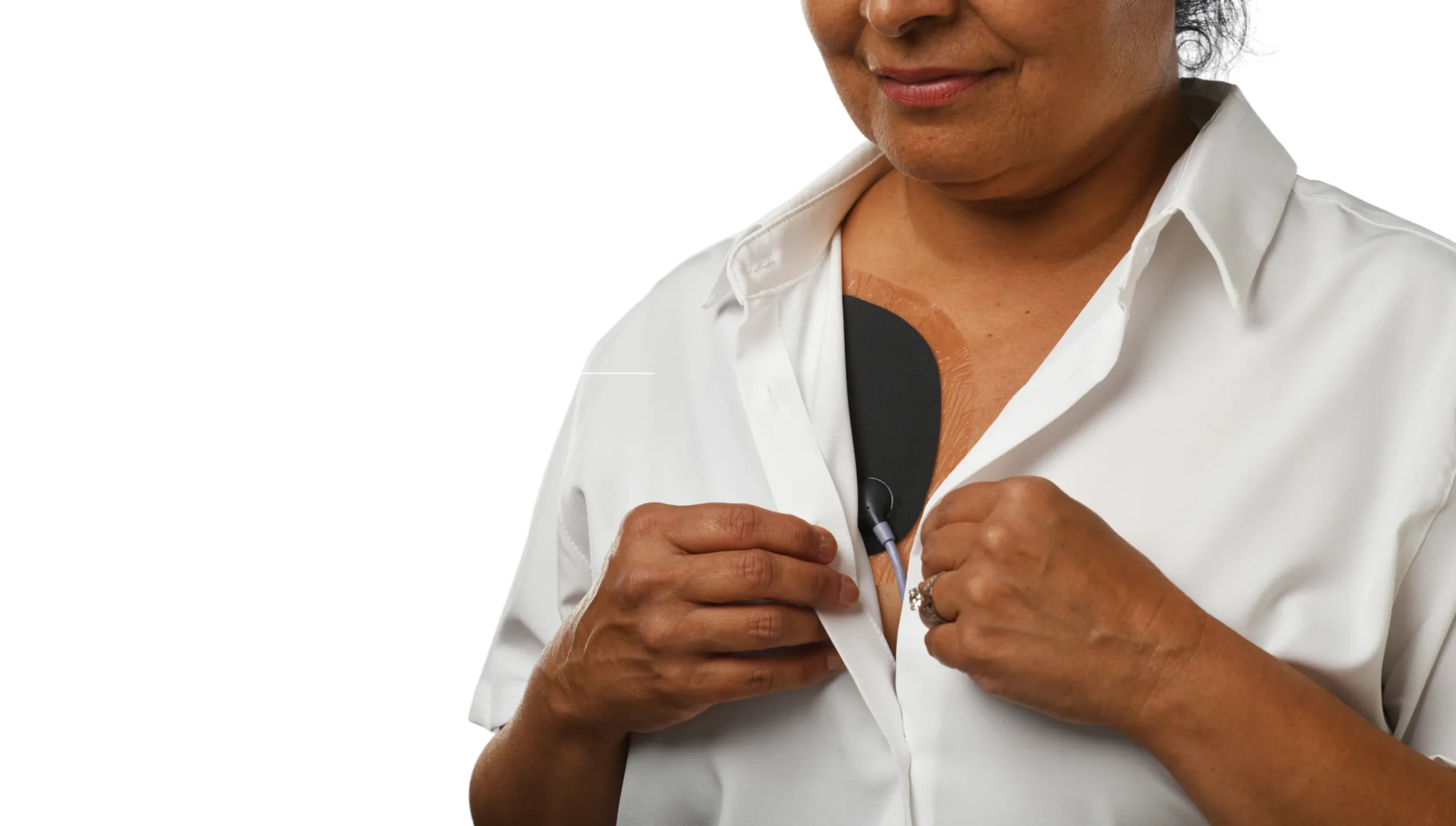
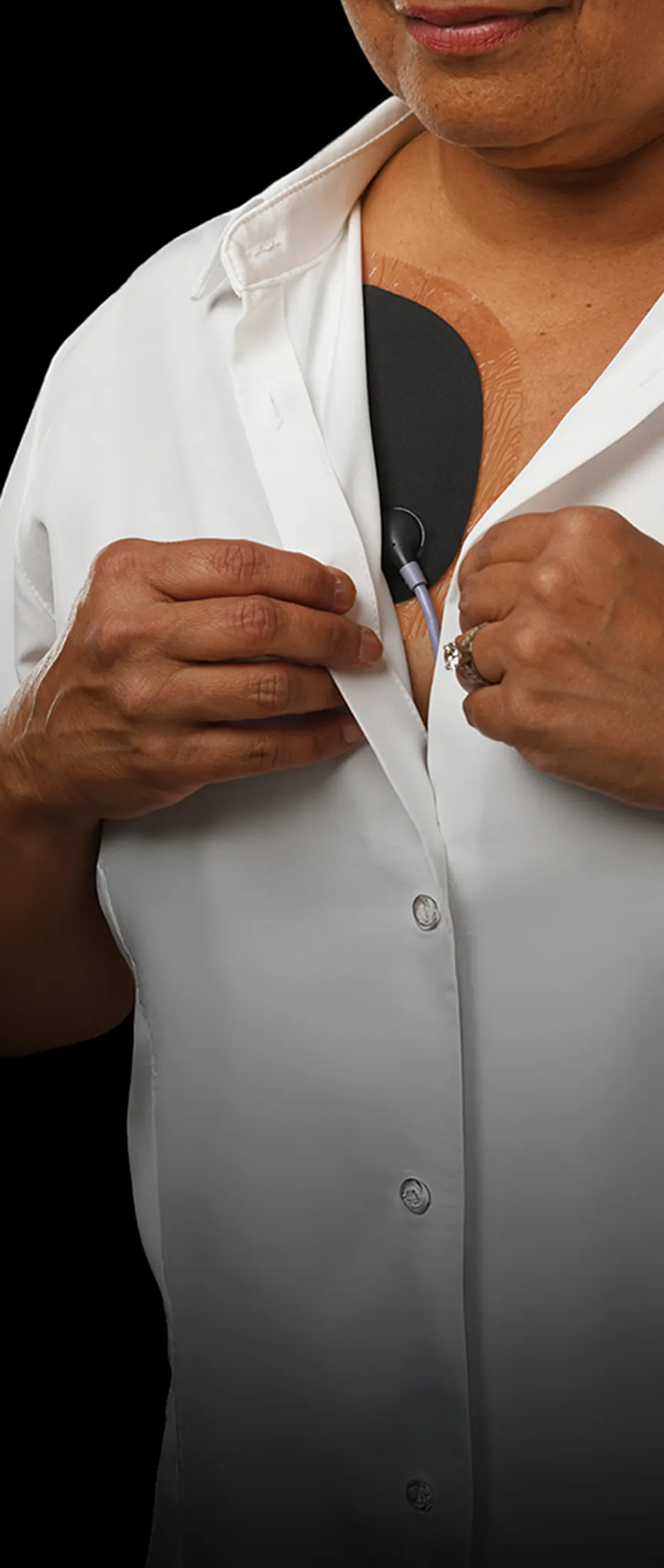
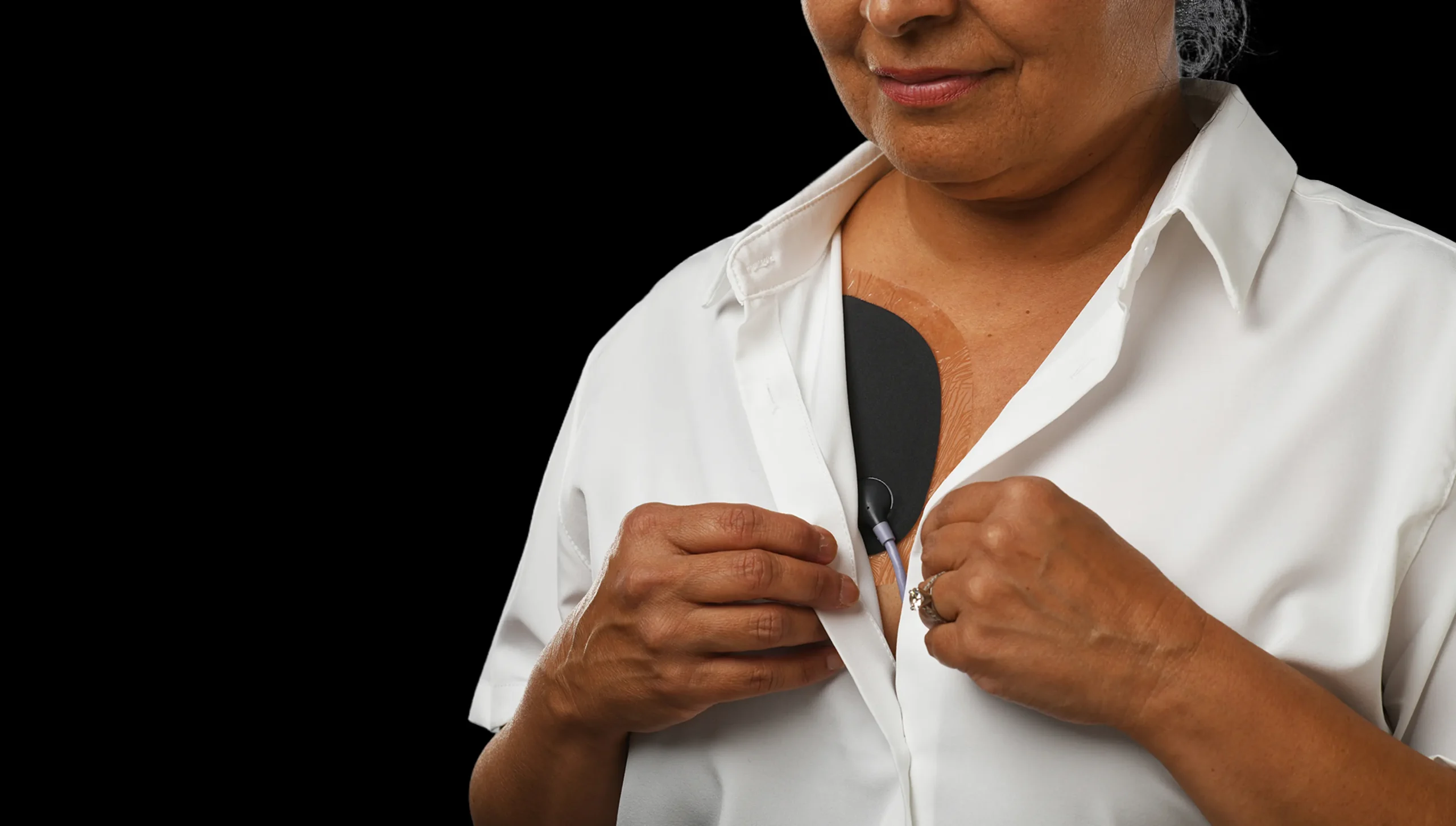
Jewel: Sleek continuous protection designed for living.
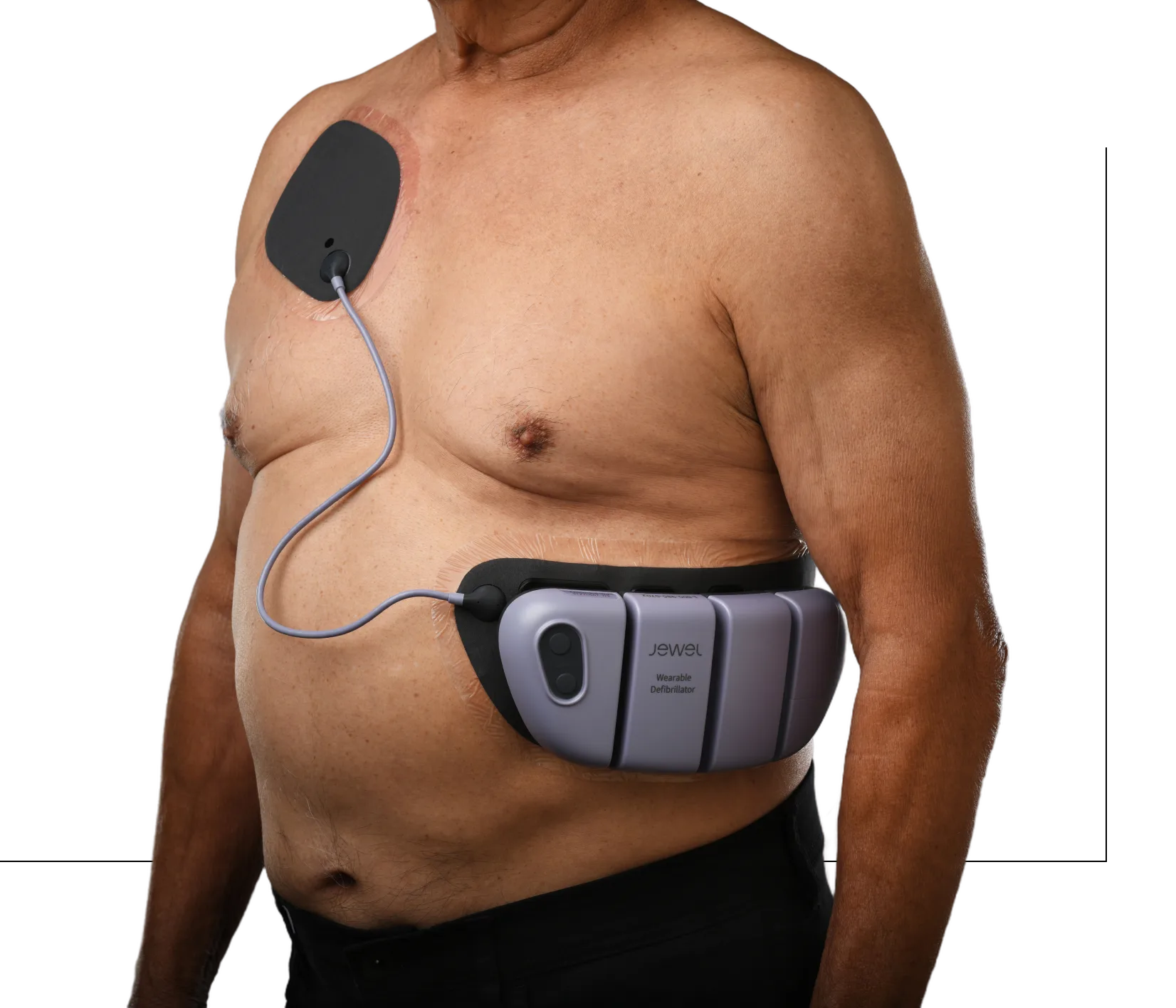
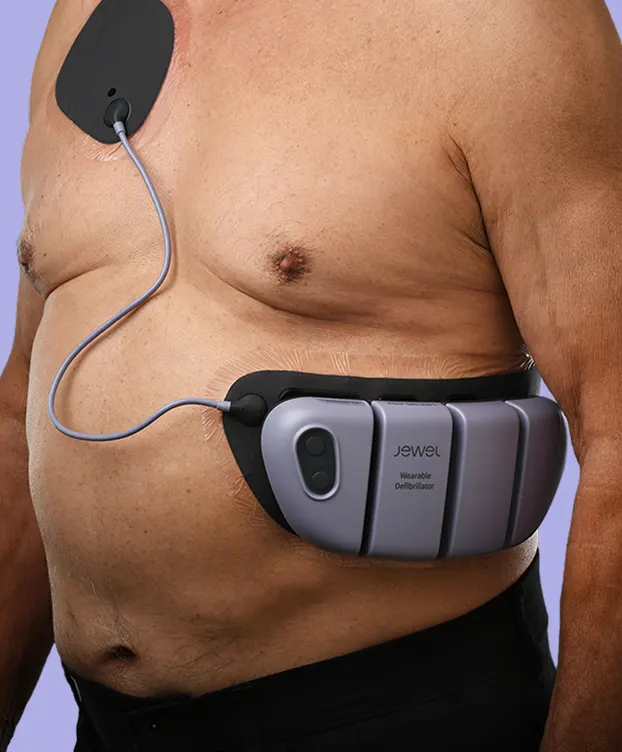
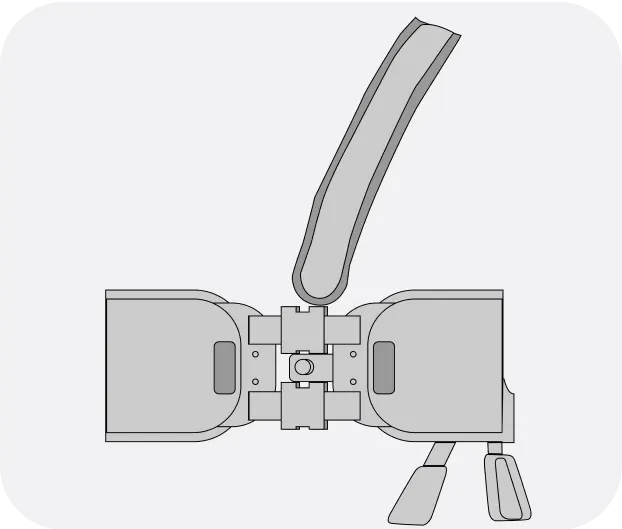
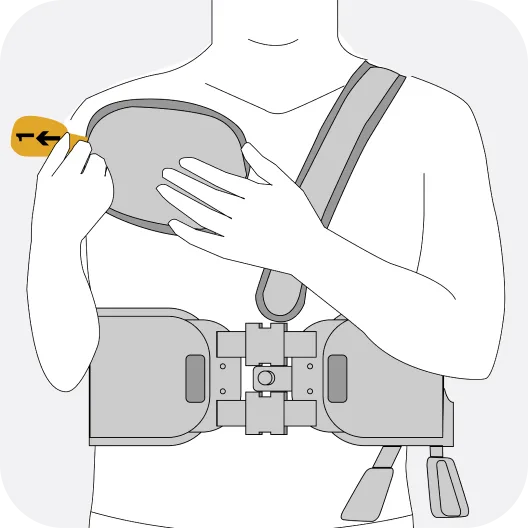
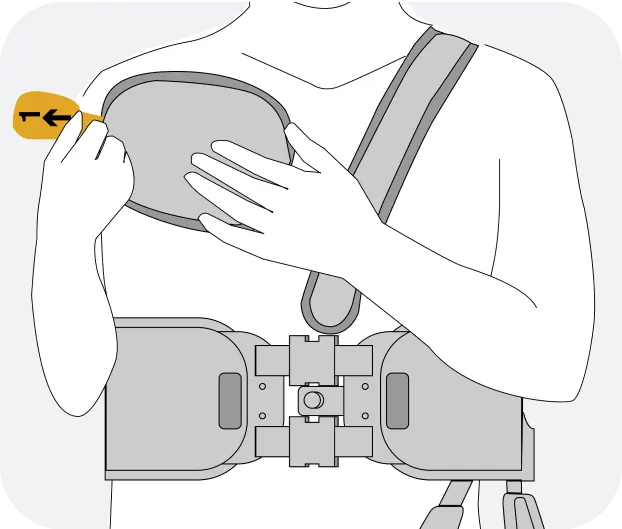
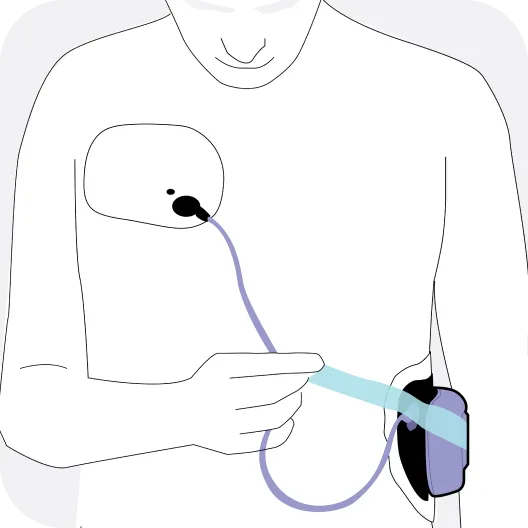
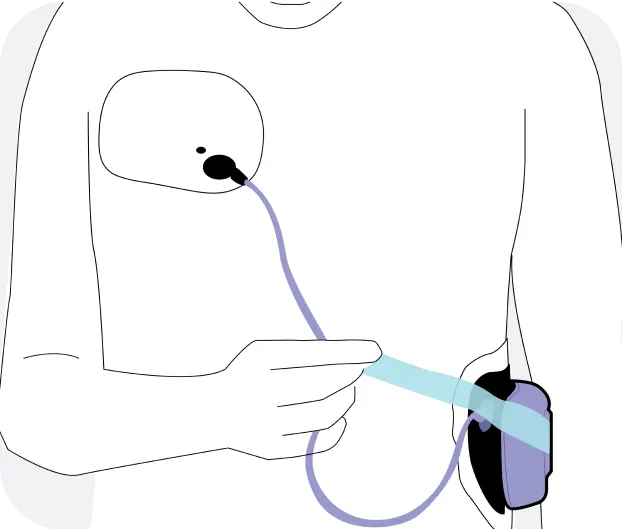
The Jewel® patch wearable cardioverter defibrillator is revolutionizing protection against out-of-hospital cardiac arrest. The device adheres to the body via two patches that only need to be replaced weekly.
The patches are connected to the Jewel monitoring & defibrillator unit, which monitors the heart’s rhythm and can deliver life-saving therapy if needed.
The first wearable patch cardioverter
defibrillator that’s truly wearable
Jewel can deliver a full week of continuous wear-time.
Jewel is:
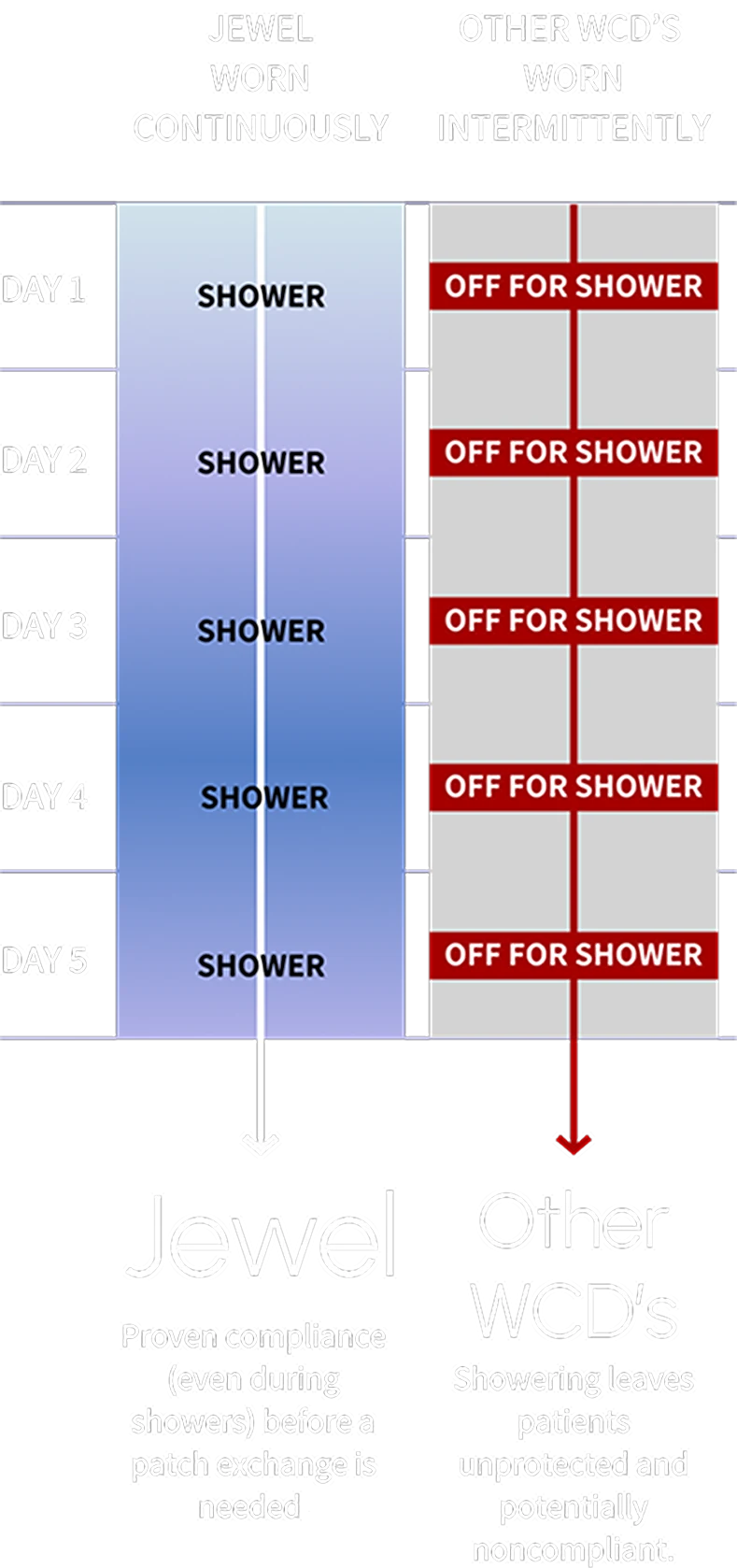
Worn continuously for up to a week at a time
Suitable for use while showering
Sleek, discreet, and comfortable to wear
Simple to use and maintain
Implementing Jewel
Clinical evidence
Jewel is a revolutionary wearable patch cardioverter defibrillator demonstrated to offer truly continuous, life-saving benefits.
The Jewel IDE
Study demonstrates
lives saved with zero deaths
High compliance and protected time
Patients using Jewel achieved a median wear-time of over 23 hours per day, showcasing its ease of use and integration into patients’ daily lives.
Safety and effectiveness
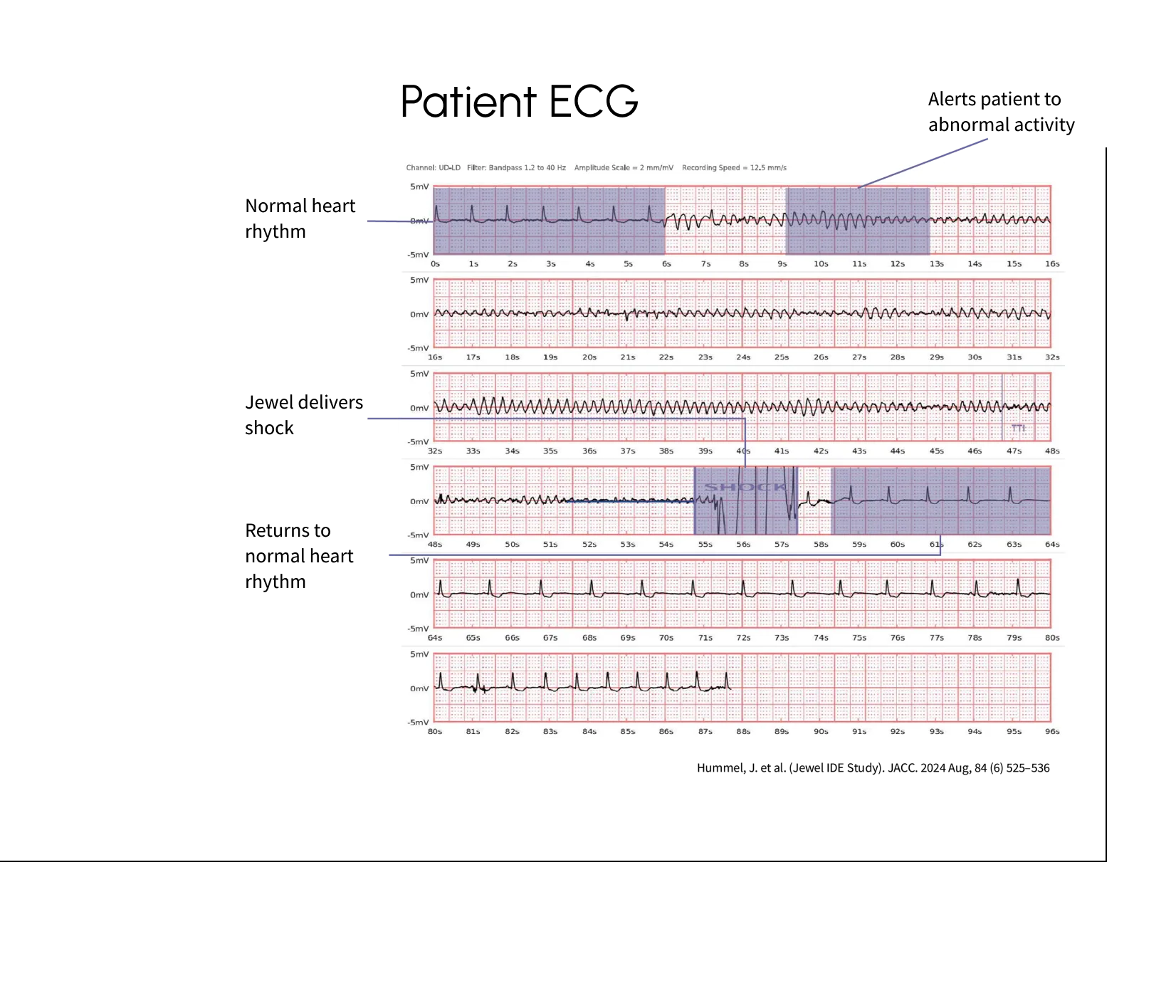
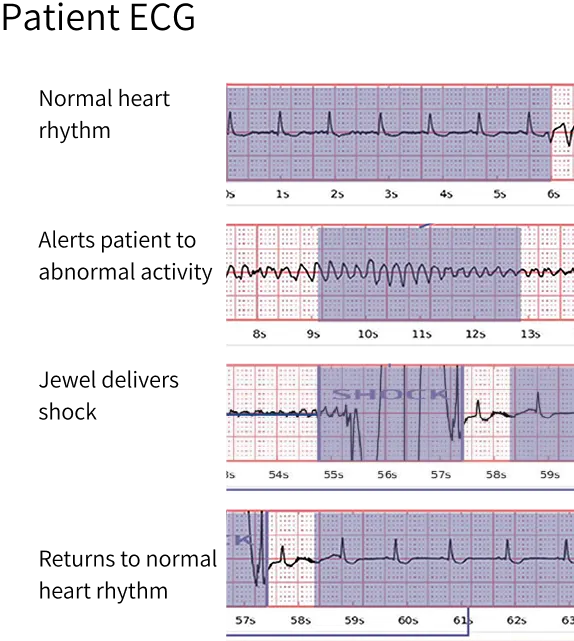
The device successfully converted eight separate ventricular tachycardia or ventricular fibrillation events in six (6) different patients, effectively preventing SCA. No deaths were observed in patients while enrolled in the study.
Low inappropriate shock rates
The study observed a very low inappropriate shock rate of just 0.36 shocks per 100 patient-months, providing patients with peace of mind.
Jewel successfully met its pre-defined endpoints.
The Jewel EP Lab Study shows the efficacy of the
Jewel defibrillator.
The first in human study
The Jewel EP Lab Study (NCT05490459) established the foundational safety and effectiveness of Jewel to identify and convert life-threatening rhythms in a single shock.
Jewel successfully met its pre-defined endpoints.
Clinical Guidelines for wearable cardioverter defibrillators
The American Heart Association, American College of Cardiology, and Heart Rhythm Society recommend wearable cardioverter defibrillators for primary prevention of sudden cardiac death in patients with ischemic heart disease.
Figure: Primary prevention of SCD in patients with ischemic heart disease.
Colors correspond to Class of Recommendation in Table 1.
See Section 7.1.2 for discussion.
*Scenarios exist for early ICD placement in select circumstances such as patients with a pacing indication or syncope
†Advanced HF therapy includes CRT, cardiac transplant, and LVAD thought due to VT.
These are detailed elsewhere in an HRS/ACC/AHA expert consensus statement.S7.1.2-24 CRT indicates cardiac resynchronization therapy; EP, electrophysiological; GDMT, guideline-directed management and therapy; HF, heart failure; ICD, implantable cardioverter-defibrillator; IHD, ischemic heart disease; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NSVT, nonsustained ventricular tachycardia; NYHA, New York Heart Association; pts, patients; SCD, sudden cardiac death; VT, ventricular tachycardia; and WCD, wearable cardioverter-defibrillator.
Circ 138(13), e272 – e391: DOI 10.1161/CIR.0000000000000549
Guidelines for patients with ischemic heart disease
The American Heart Association, American College of Cardiology, and Heart Rhythm Society recommend wearable cardioverter defibrillators (WCDs) for secondary and primary prevention of sudden cardiac death in patients with nonischemic cardiomyopathy.
Figure: Secondary and primary prevention of SCD in patients with NICM.
Colors correspond to Class of Recommendation in Table 1. See Section 7.2 for discussion. *ICD candidacy as determined by functional status, life expectancy or patient preference. 2° indicates secondary; EP, electrophysiological; GDMT, guideline-directed management and therapy; HF, heart failure; ICD, implantable cardioverter-defibrillator; LVEF, left ventricular ejection fraction; NICM, nonischemic cardiomyopathy; SCA, sudden cardiac arrest; SCD, sudden cardiac death; VA, ventricular arrhythmia; and WCD, wearable cardioverter-defibrillator.
Circ 138(13), e272 – e391: DOI 10.1161/CIR.0000000000000549
Guidelines for patients with nonischemic cardiomyopathy
The American Heart Association, American College of Cardiology, and Heart Rhythm Society all recommend
wearable cardioverter defibrillators for certain patients at high risk of sudden cardiac arrest.
Figure: guideline recommendation for wearable cardioverter defibrillators
Circ 138(13), e272 – e391: DOI 10.1161/CIR.0000000000000549
Jewel is easy
to order.
Contact your Jewel team at Element Science anytime for order support, verbal orders, or any other questions you may have.
We ensure
patient success.
We work with patients every step of their journey with Jewel, beginning with the very first visit and continuing until they no longer need Jewel’s protection.
Patients leave confident in how to use Jewel.
Patient fitting and training session
Hands-on Introduction To Jewel
Placement Accessory Fitting
Training On Jewel Application
Training On Jewel Removal
Automated support
On-demand
support
Customer service
Digital and print
(Patient guide, eIFU, and
training videos)
We empower your patients every step of their journey with Jewel, beginning with the very first visit and continuing until they no longer need Jewel’s protection.
Planned outreach by our team ensures patient compliance and confidence.
You’re never alone, so no issues impact wear time and protection.
24-hour support is available if your patient ever has a concern.
Have more questions?
Please answer the following questions in the form to the right to get started.
FAQ
for providers
What is Jewel?
Jewel is a revolutionary wearable cardioverter defibrillator that redefines living with life-saving technology. Designed to be easier to wear, Jewel offers continuous protection to patients as they transition from at-risk to recovery.
Why did Element Science decide to redesign the wearable cardioverter defibrillator?
Only 110,000 of 355,000 people who are discharged with a high risk for sudden cardiac arrest receive a wearable cardioverter defibrillator. Yet, with traditional wearable cardioverter defibrillators, there are stills deaths due to compliance issues and the need to take them off for maintenance, which leaves patients unprotected. Jewel was designed to be easier to wear and to be worn continuously without daily maintenance or interrupting daily living, so patients can be protected for more time.
Which patients are eligible for Jewel?
The Jewel Wearable Patch Defibrillator is indicated for adult patients 18 years of age and older who are at high risk for sudden cardiac arrest and either are not candidates for or refuse an implantable defibrillator. It can also be used as a transitional therapy for patients awaiting long-term treatment.
What makes Jewel different?
Jewel is specifically designed to address the limitations of traditional wearable cardioverter defibrillators. It can be comfortably worn for up to a week at a time (even in the shower) for continuous protection and compliance. Our concierge support is always there, ready to help ensure patients continue to wear Jewel for the duration of their prescription period.
Who can order Jewel?
Jewel must be ordered by a licensed healthcare provider with prescriptive authority in the state in which they will be prescribing Jewel. This may include physicians, nurse practitioners, or physician assistants.
What clinical data supports the use of wearable cardioverter defibrillators (WCDs)?
There is a body of clinical data on wearable cardioverter defibrillators (WCDs) that highlights the life-saving benefits of the technology if patients wear the device and achieve high daily protected time.
What clinical data supports the use of Jewel specifically?
Multiple clinical studies have shown Jewel® is a revolutionary wearable cardioverter defibrillator with life-saving benefits because it’s easier to wear and offers truly continuous protection.
Is Jewel included in any cardiovascular care guidelines?
Yes, the American Heart Association (AHA) recommends wearable cardioverter defibrillators (WCDs) for certain patients at high risk for sudden cardiac arrest.
Is Jewel FDA approved?
Yes, Jewel received FDA approval on ________ 2024
What support is available for patients using Jewel?
We engage patients at multiple touchpoints to ensure they are confident using Jewel. Our trained staff introduce patients to Jewel and provide a personal fitting so the device can offer maximum comfort and protection. Patients learn how to apply and remove Jewel properly.
Our team connects with patients at planned intervals to check on their experience and ensure they feel confident and protected.
All patients have access to 24-hour support teams that can answer their questions about Jewel, from proper placement and removal to the Jewel App and even skin health.
Where is Jewel available?
To find out if Jewel is available in your area, please contact us:
- Phone: 1-800-985-5702
- Email: customerservice@elementsci.com
- Contact us
Can I speak with a representative?
Yes, representatives are available 24 hours per day, 7 days per week. You can reach us by:
- Phone: 1-800-985-5702
- Email: customerservice@elementsci.com
- Contact us